#R
-
CXR
Terms (Fleischner 1 based. Fleischner 2 is more focused on CT terms)
Optical Density (aka the amount of blackening): factor of amount of tissue and physical density of tissue the photon must pass through, leading to attenuation of strength (= whiter) => lower optical density = an opacity
Infiltrate: something (e.g. cells, fluids) in the lung in greater than expected amounts. This is a pathologic diagnosis. Not to be used in radiology.
Atelectasis: incomplete expansion. Evidence of diminished volume that may or may not influence lucency. There are direct signs ( ) and indirect signs ( ).
- Quantified as total pulmonary, lobar, segmental, subsegmental (=discoid, platelike).
- Obstructive (bronchial) vs non-obstructive
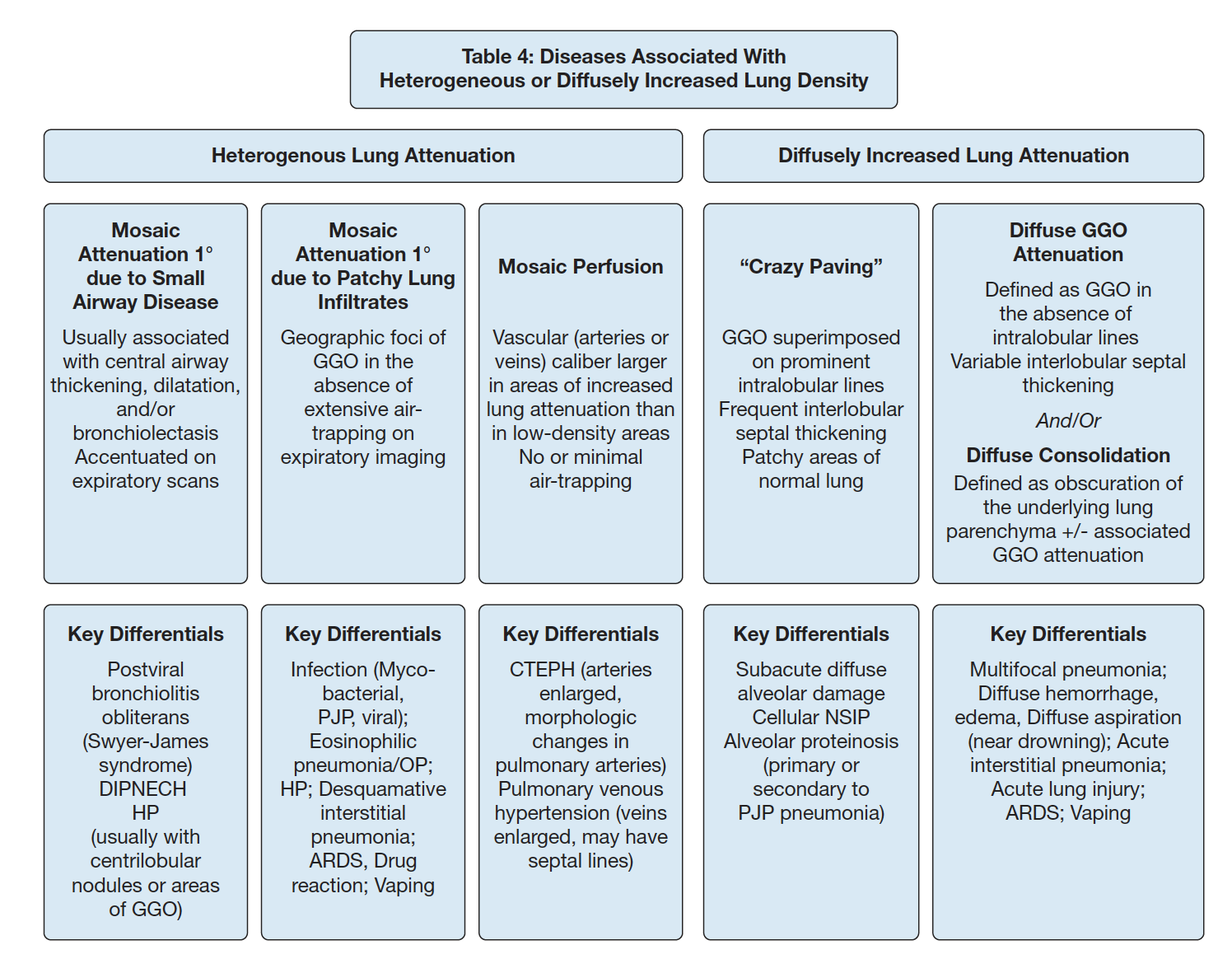
Air retention: excess retention due to closure of the airway during expiration (dynamic). Not to be used to refer to hyper-inflattion at TLC. One example of evidence of this is mosaic attenuation.
Consolidation: air in lung is replaced by products of disease (which is an inferred conclusion in a clinical context). Transudate or exudate (water, pus, blood...). Sometimes (not always) keyed off by air bronchograms.
- can describe extent (subsegmental, segmental, multisegmental, lobar)
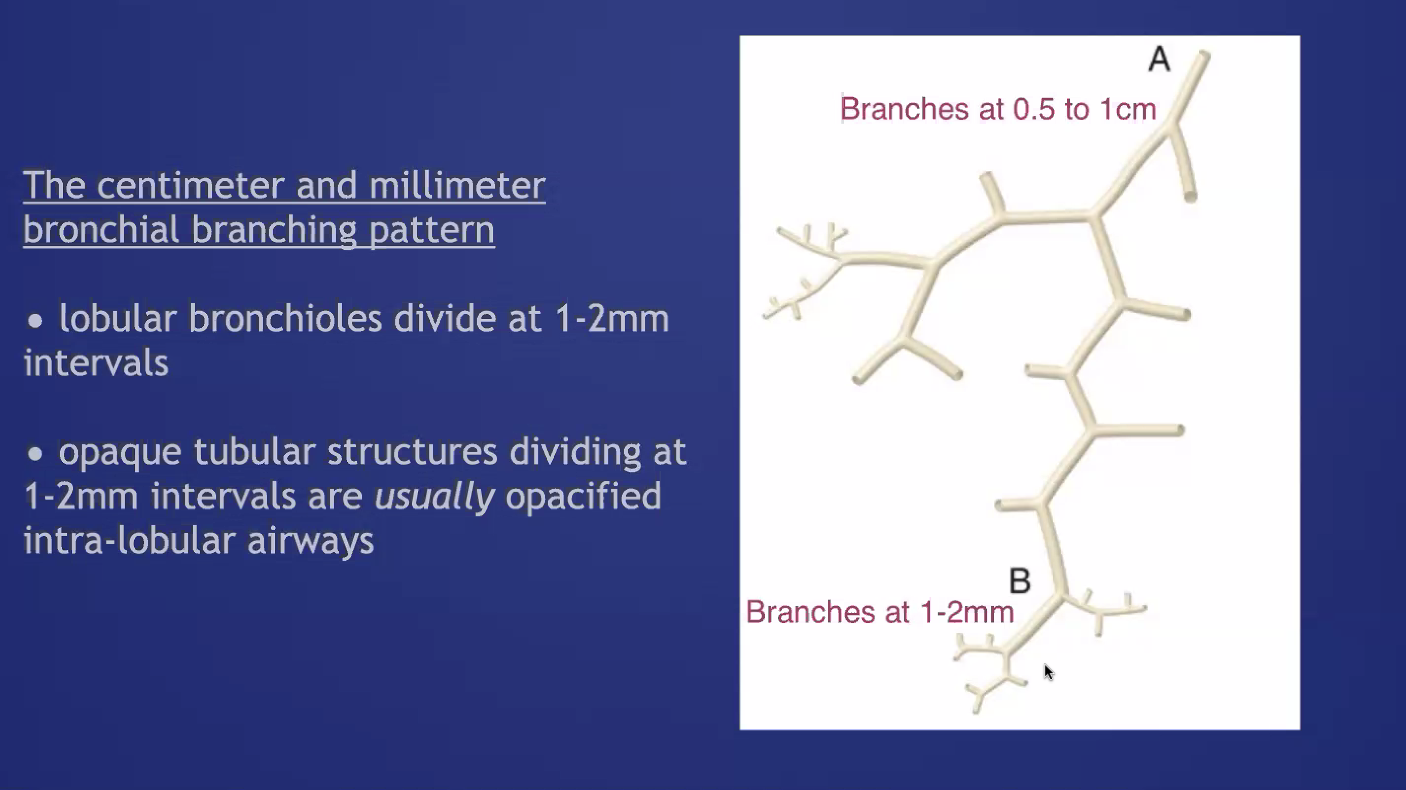
Air Bronchogram: air filled bronchus (peripheral to hilum) surrounded by airless lung. Does not necessarily imply consolidation (because it could be due to atelectasis. can also be in interstitial disease such as sarcoid)
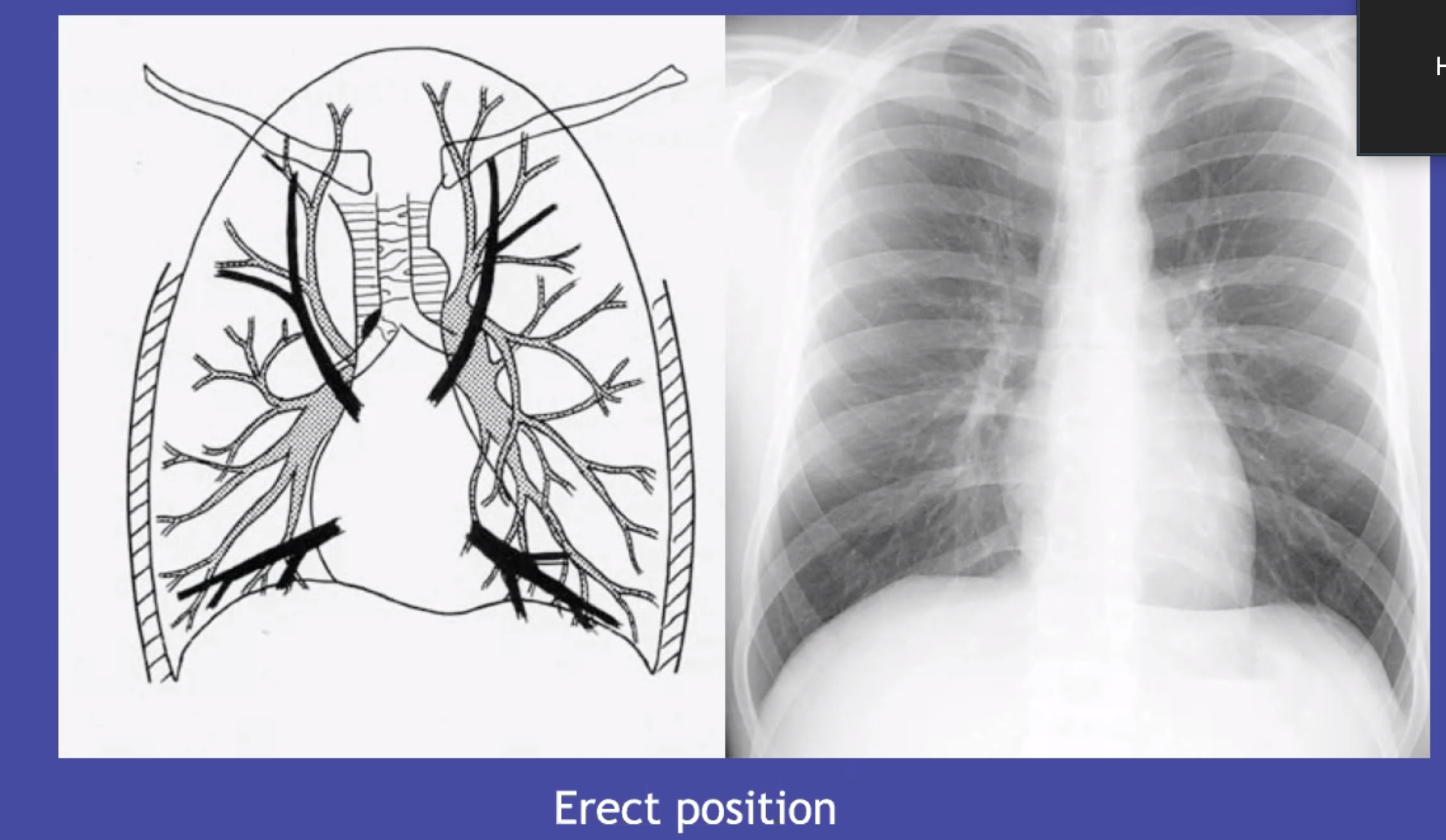
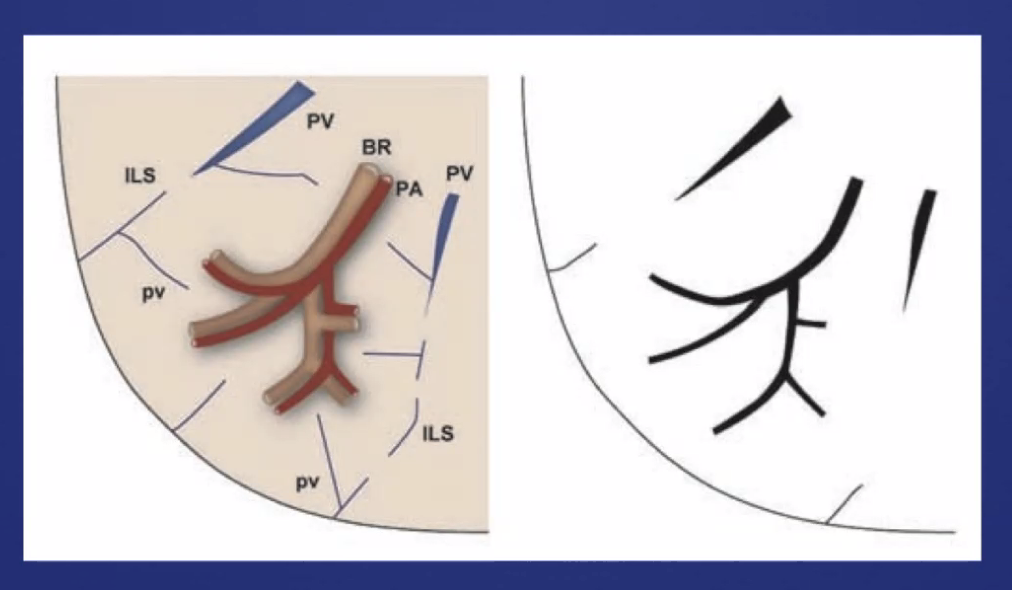
Pulmonary vessels: caliber, compair upper and lower, look at branching, try to distinguish arteries and veins
- R upper hilum: superior vein is lateral to adjacent pulmonary artery.
- pattern is normal, everted (upper are larger, chronic pulmonary venous hypertension), or balanced (equally distended in upper and lower, acute fluid overload)
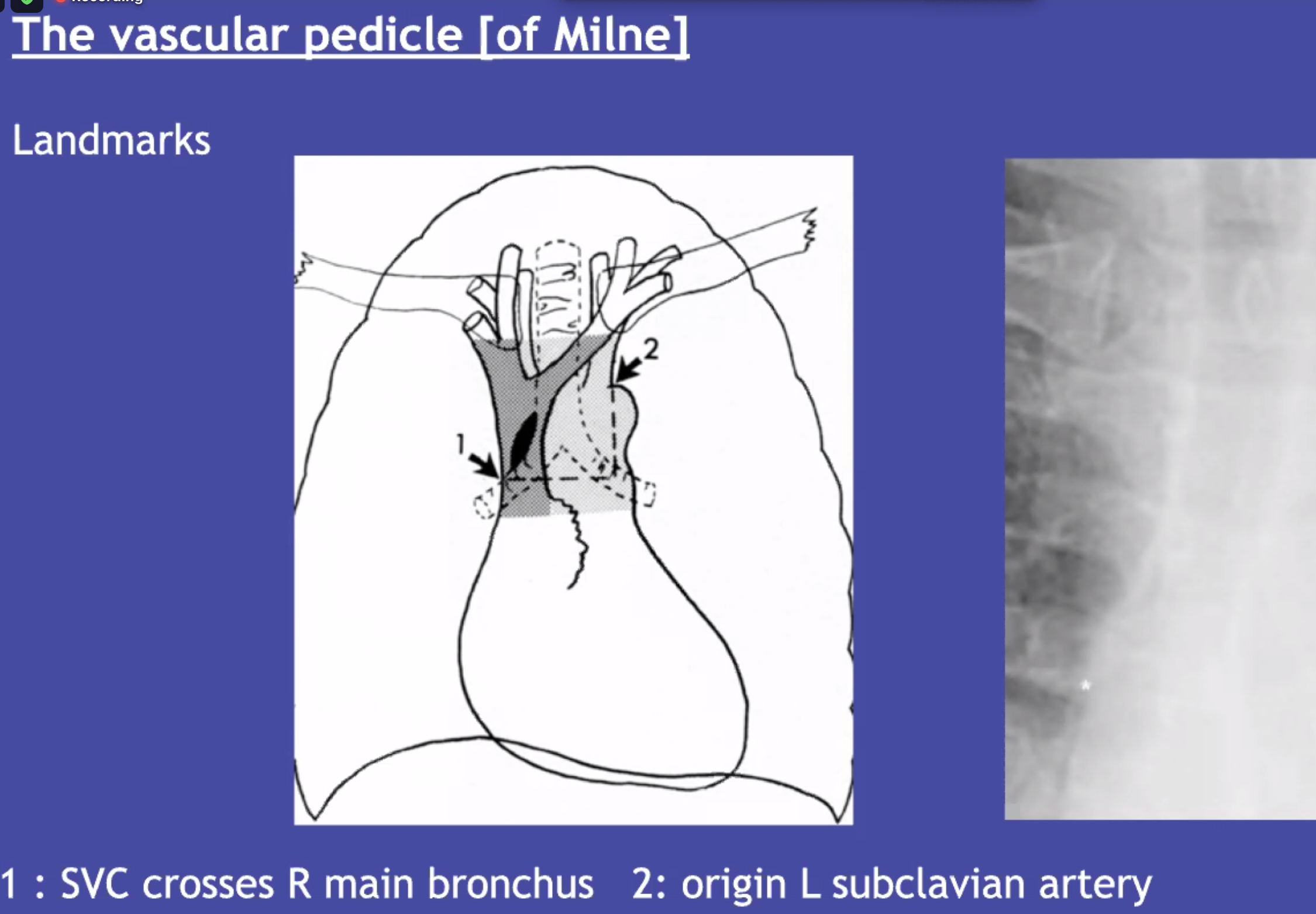
Vascular pedicle: distensible (SVC and azygous vein) that can give you an indication about the CVP. (wider = suggested elevated SVC pressure)
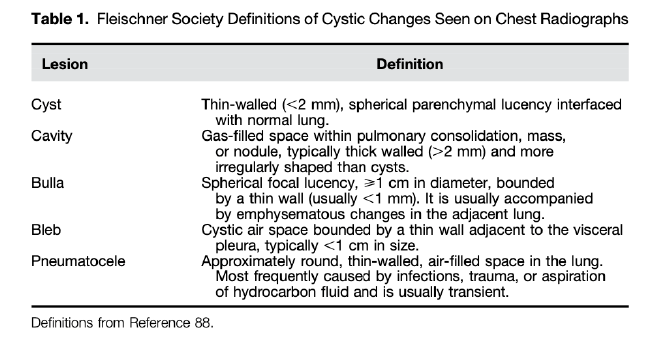
Cyst: circumscribed space, thin wall, 1cm or larger.
Bulla: any sharply demarcated lucency, greater than 1cm. No 'wall' around it (differentiates from cyst). There is still some remnant tissue present.
####Descriptors of Opacification:
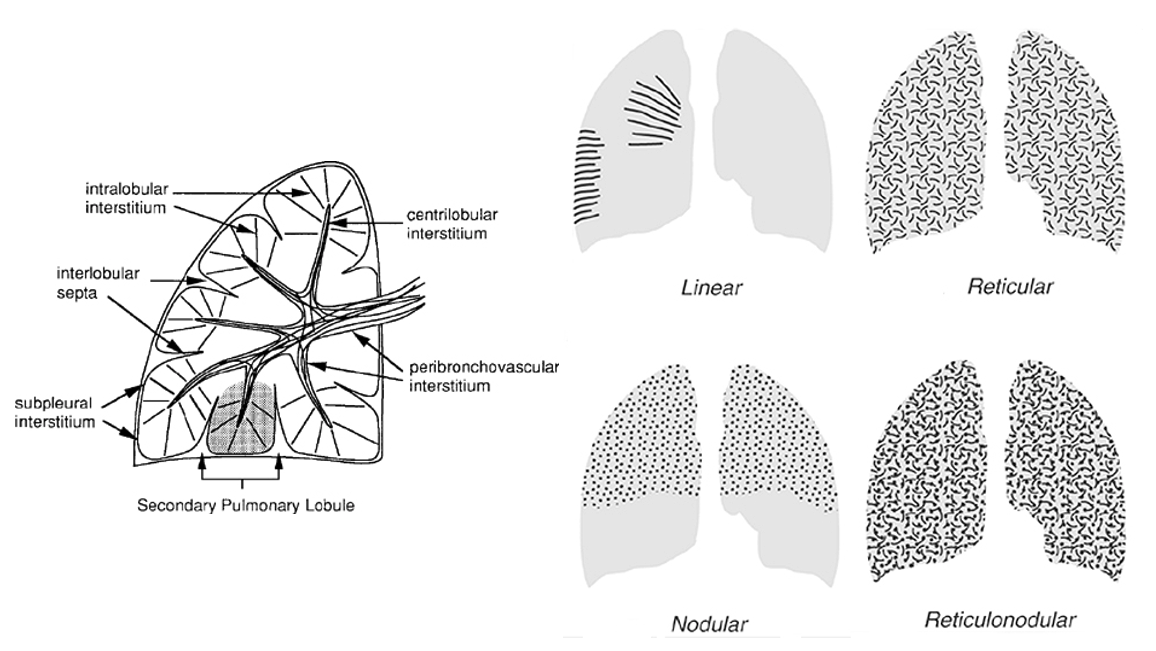
Nodular: a collection of small, roughly circular, discrete, 2-10mm opacities without marginal spiculation. Generally uniform in size. Miliary pattern if smaller than 2mm (the radiographic appearance is caused by a summation effect).
Reticular: numerous irregular (non-round) opacities that together resemble a net. No pathologic connotation (not a synonym for interstitial disease of the lung). Often reflect insterstitial inflammation/fluid/blood, whereas linear opacities are more likely to reflect peribronchial inflammation.
Reticulonodular: combination of the two patterns. No pathologic connotation.
Inflation: state of being expanded with gas, Aeration: to be full of air. (Don't say expiratory or inspiratory - radiographs don't breath)
Vascular prominence: increase in caliber or number (though, caution because the expected range of normal is large)... because this is noncommital, it probably should be avoided.
###Adequacy:
Want 9 ribs. Less than 8 = poor inspiration. Penetration? should be able to see intervetebral disc spaces. Can't = under-penetrated.
Lateral XR
Left lateral conventional
R side closer = appear more divergent
Mediastinal fat = often takes up space of the retrosternal clear space.
Retro tracheal space - Raider's triangle: trachea, spine, aortic notch - vascular path
CT
Reference article in Chest: CHEST 2020; 157(3):612-635
". In 1967, Sir Godrey Hounsfield and Allan Cormack, winners of the 1979 Nobel prize in medicine or physiology for their work, used pattern recognition, x-rays taken from various angles and rotation, and a computer to analyze readings to invent the CT scanner at Electrical and Musical Industries (EMIs) Central Research Laboratories. The first patient brain CT scan was performed in 1971 in Wimbledon, United Kingdom (33). As a bit of trivia, the music and electronics company, EMI, used monies from the sales of their successful recording artists, “The Beatles,” to fund the project that developed the CT scan."
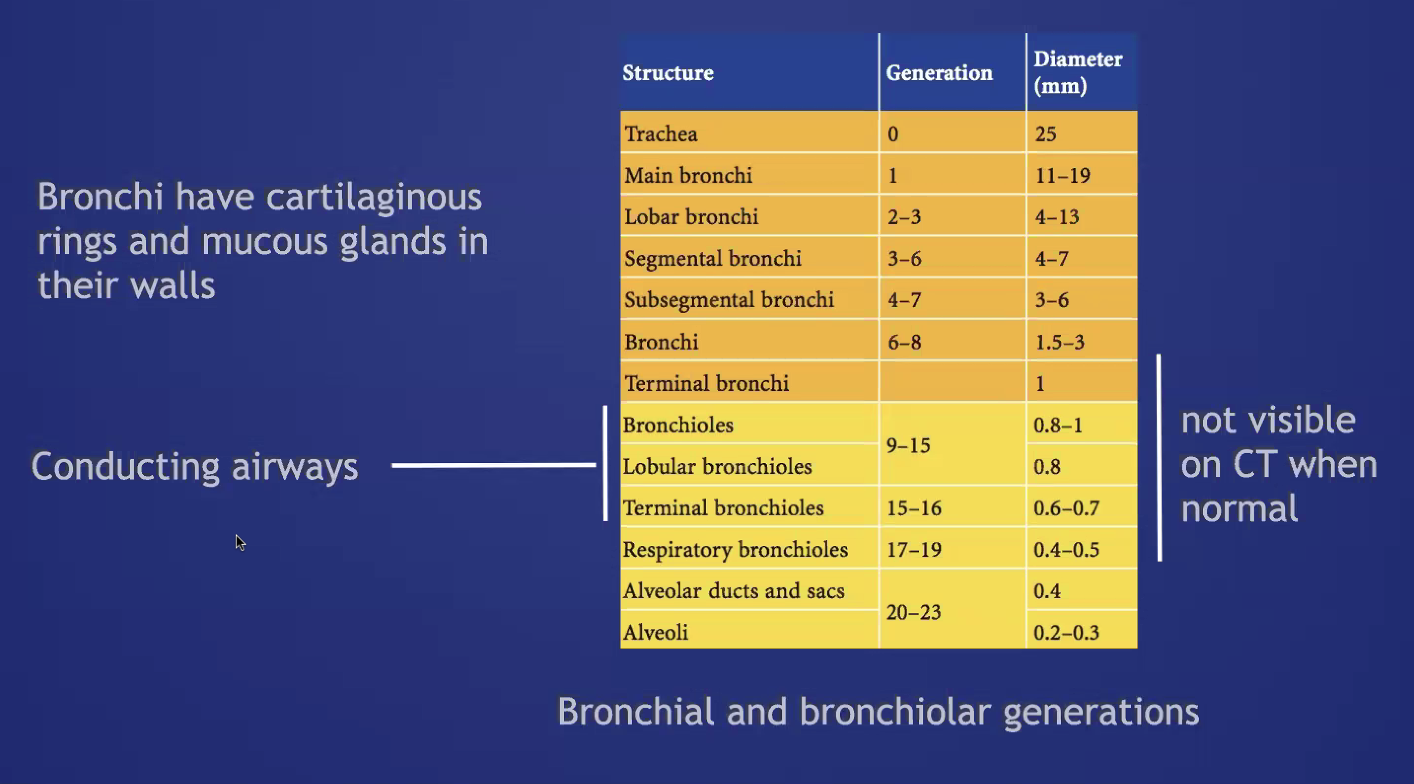
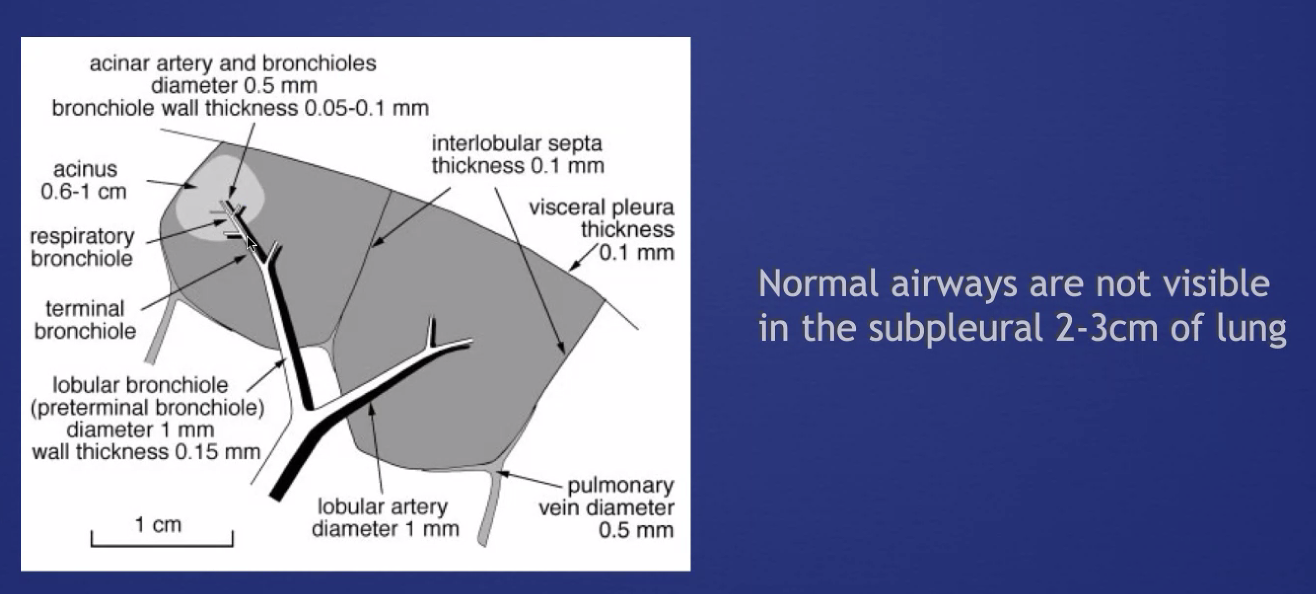
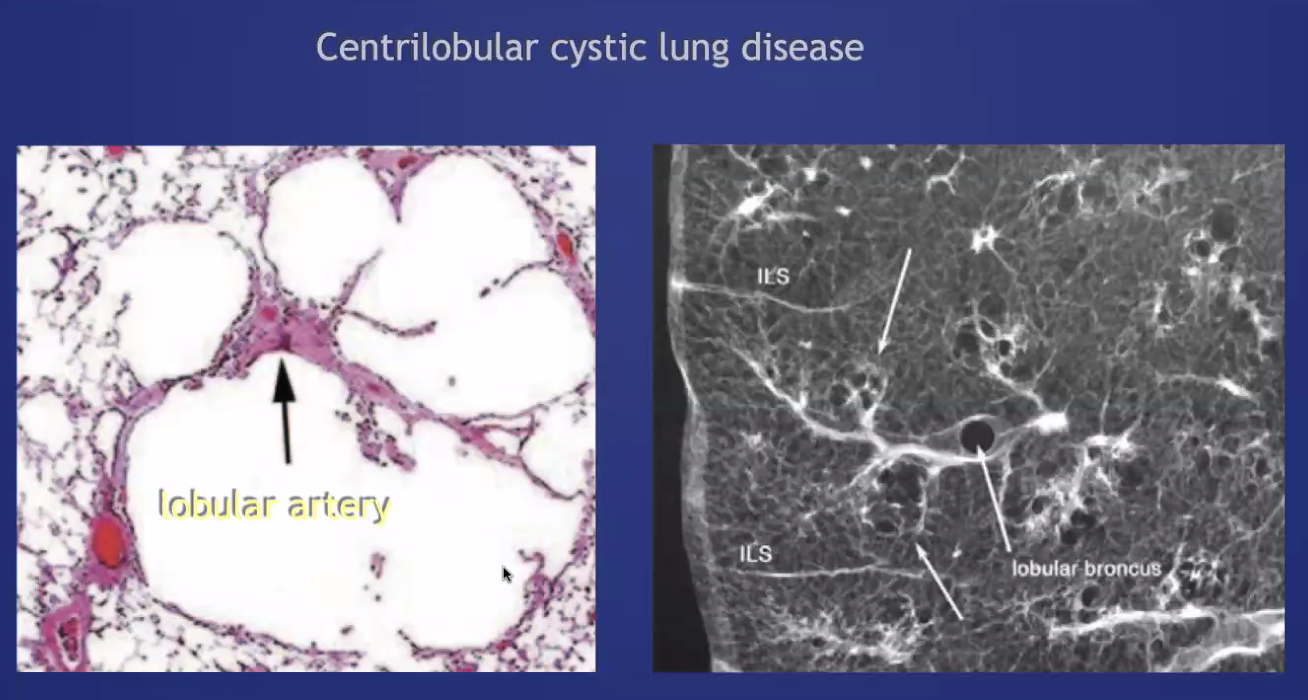
###Anatomy: (lobule level)
-There are vessels both in the septa between lobules (veins) and interlobular (artery) -Airway tracks with arteries in the CENTER of the lobules -Veins track from septa. PERIPHERY of lobules -Lymphatics track with bronchovascular bundles AND fissures.
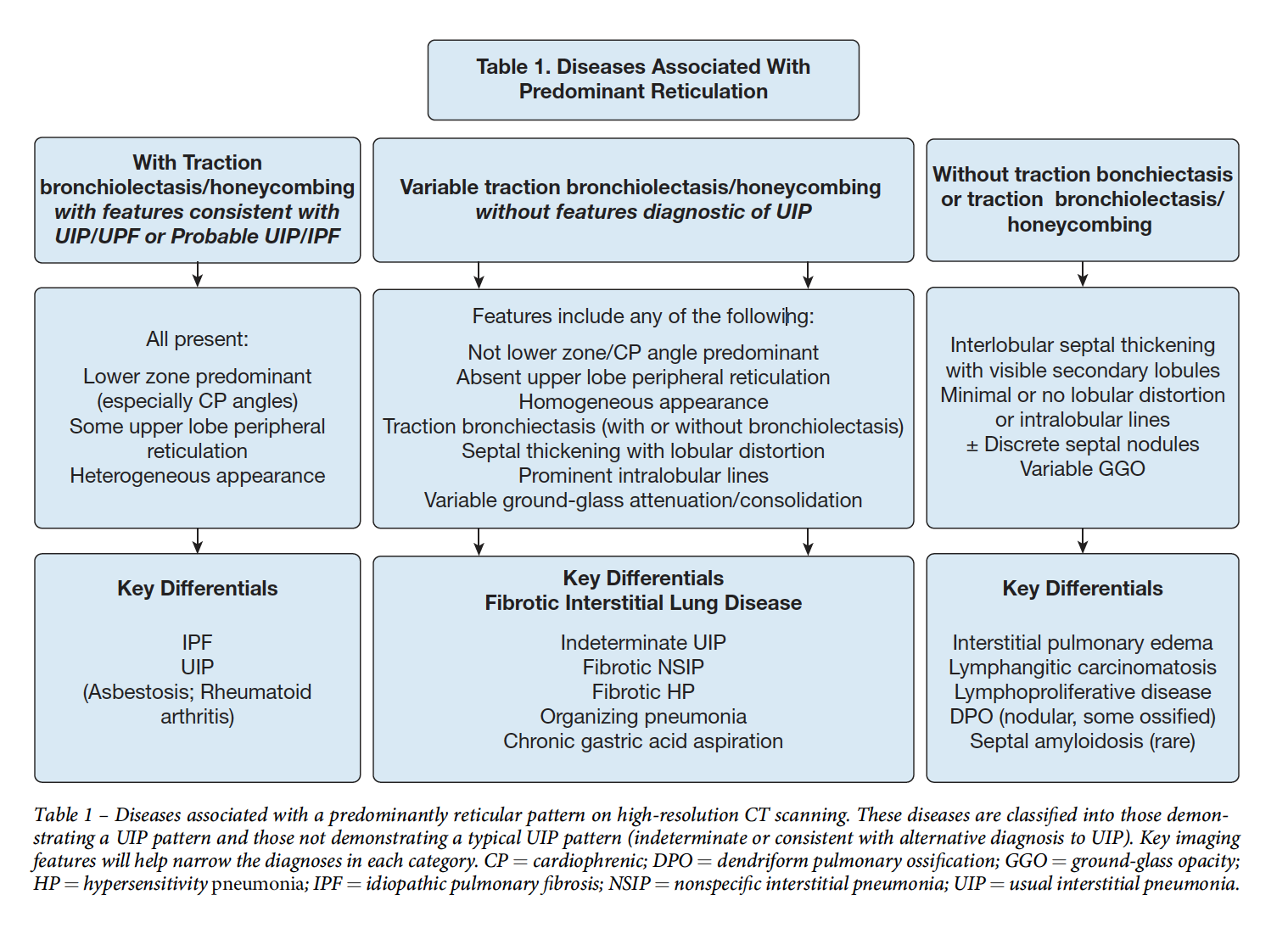
Leads to different patterns: e.g. UIP = peripheral portion of lobule fibrosis Fibrotic HP = central (bronchovascular) distribution of fibrosis, at the level of the lobule
General Approach
from CHEST 2020; 157(3):612-635
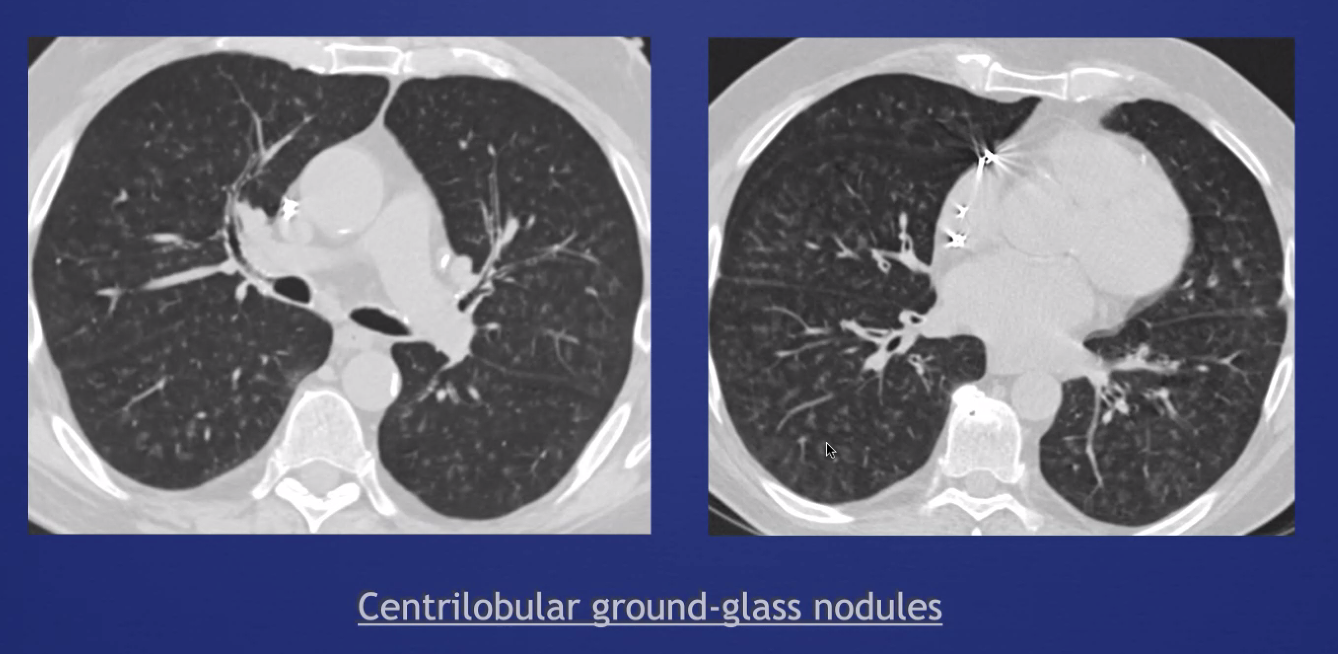
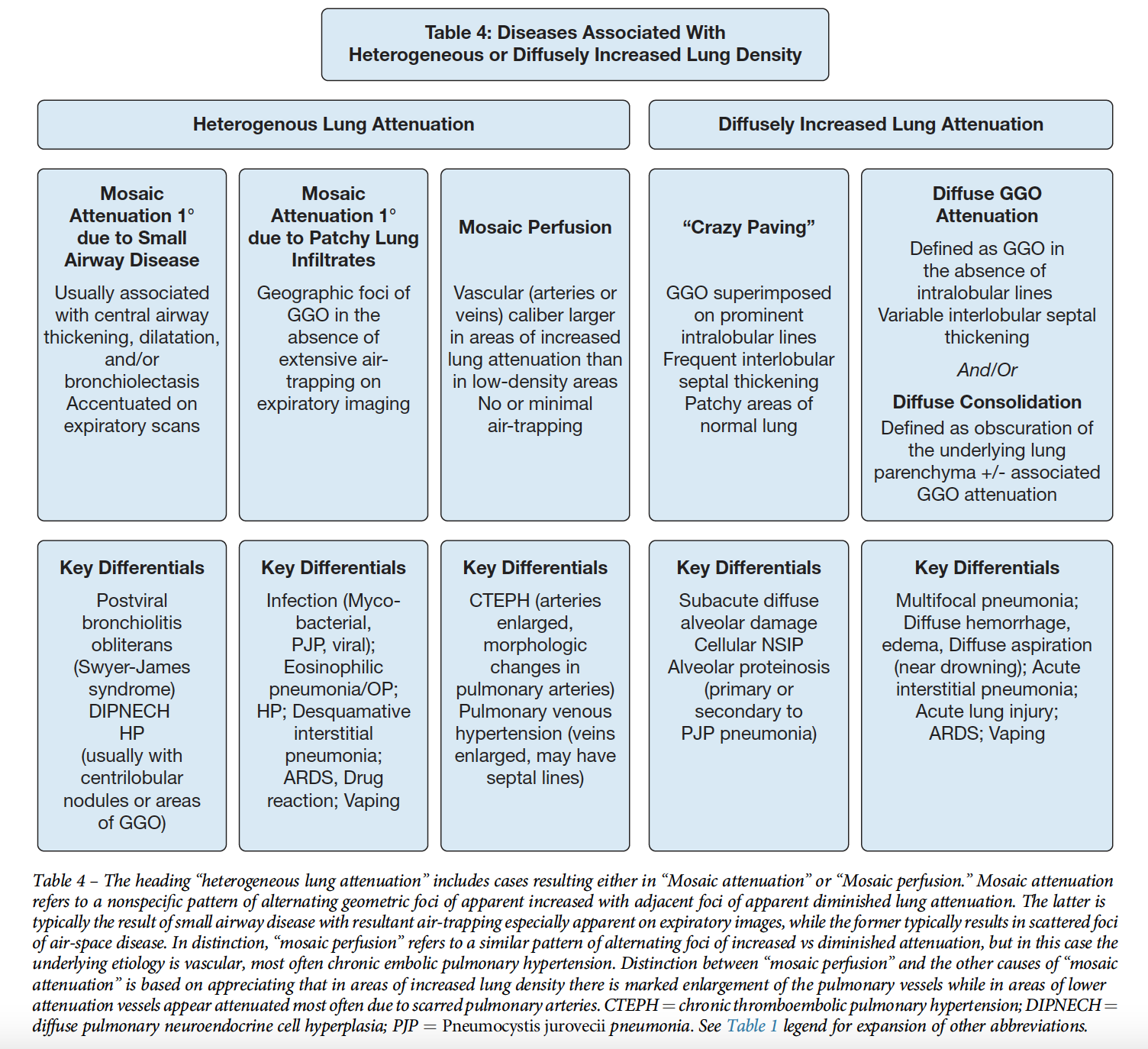
Classification of Centrilobular Abnormalities
-GGOs (either centriacinar or confluent): vessels or accumulation of cells -Bronchiolar tree in bud -Arterial tree in bud
Bronchiectasis
- Cystic
- Varicoid
Atelectasis vs Consolidation
On contrasted CT scan - uniform enhancement in atelectasis, compared to heterogenous enhancement in a consolidation. If non-contrasted, volume loss (direct or indirect signs) is crucial. Air bronchograms can occur in either.
Signs of Fluid overload
Septal thickening (veins run between lobules, in the septa) Will often be associated with pleural and fissures fluid. Also, sub pleural edema (opacification)
Ground Glass
Partial obscuration of underlying structure of the lung.
GGOS: if they are centrilobular, they will not have discrete borders (because that occurs at a septa between lobules). 'Smudges' are a hint.
As the process fills the entire lobule, it'll become confluent ('geographic opacity')
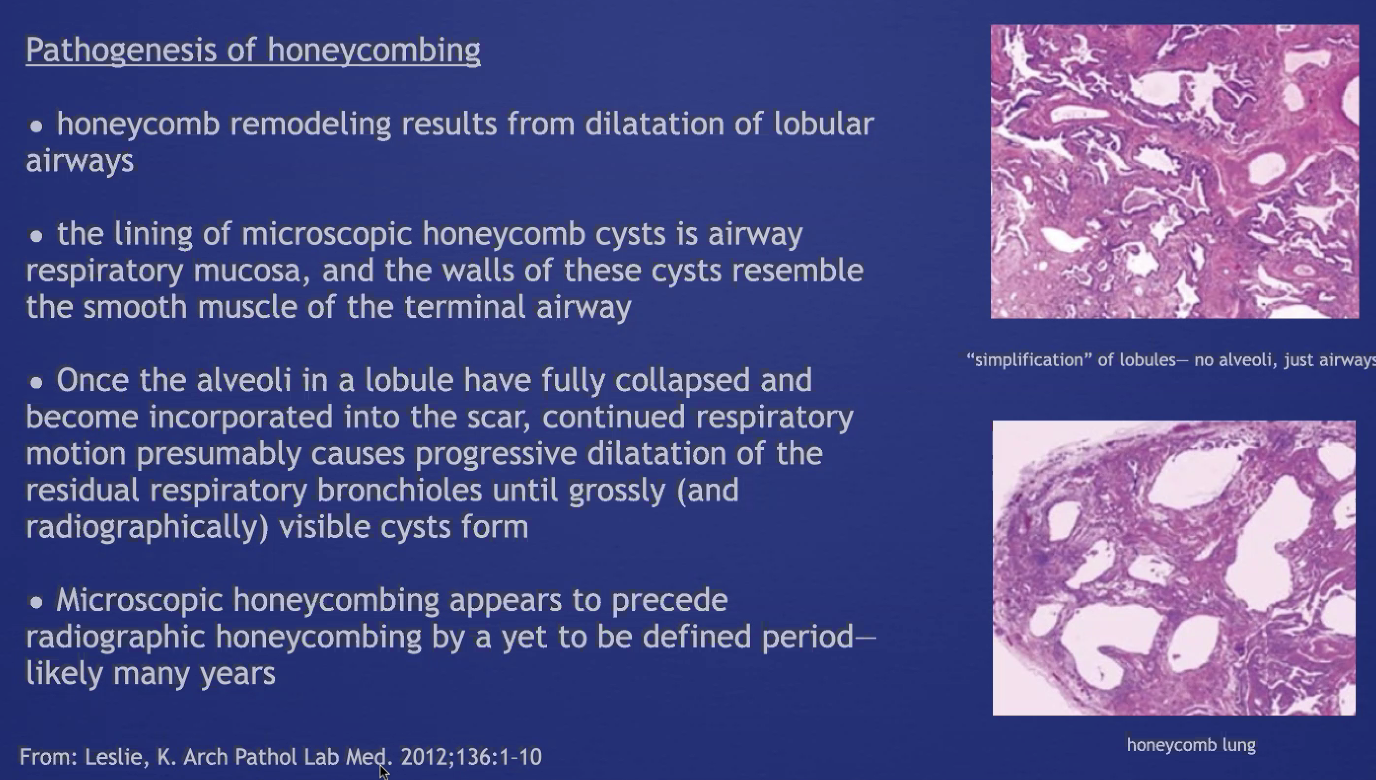
Honeycombing
A number of closely approximated ring shadows representing air spaces with walls 2-3 mm thick.
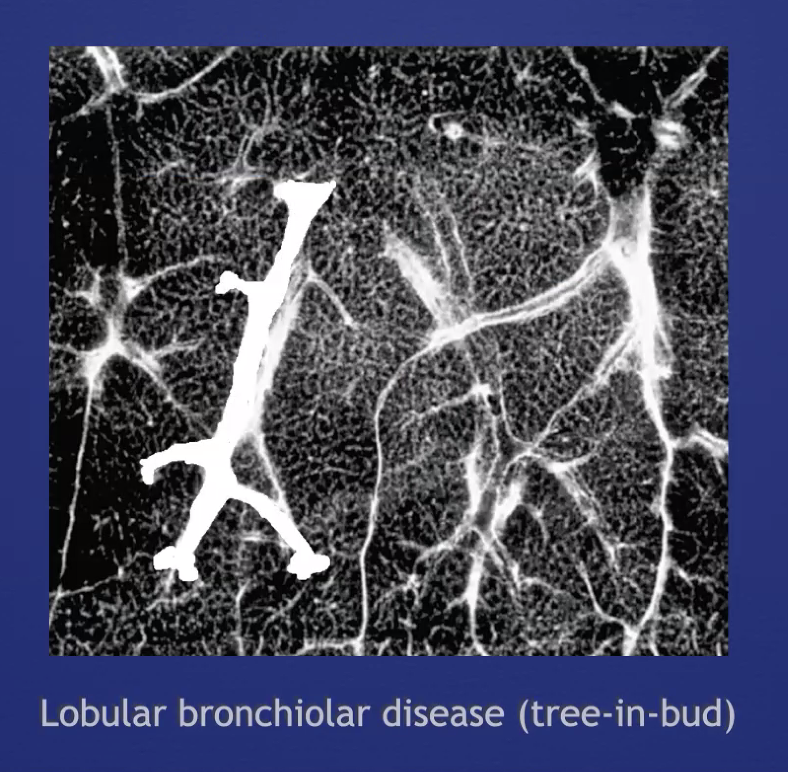
Tree in Bud
Nodular opacities in the airways at the size of bronchioles. Mostly just tree (there's often not much 'bud'). These occur in the middle of the lobule, and are the result of either arterial or airway pathology (more commonly airway)
- most often infectious bronchiolitis or aspiration bronchiolitis
- other ddx includes mucus plugs, (CF, mucostasis), follicular bronchiolitis (e.g. CVID)
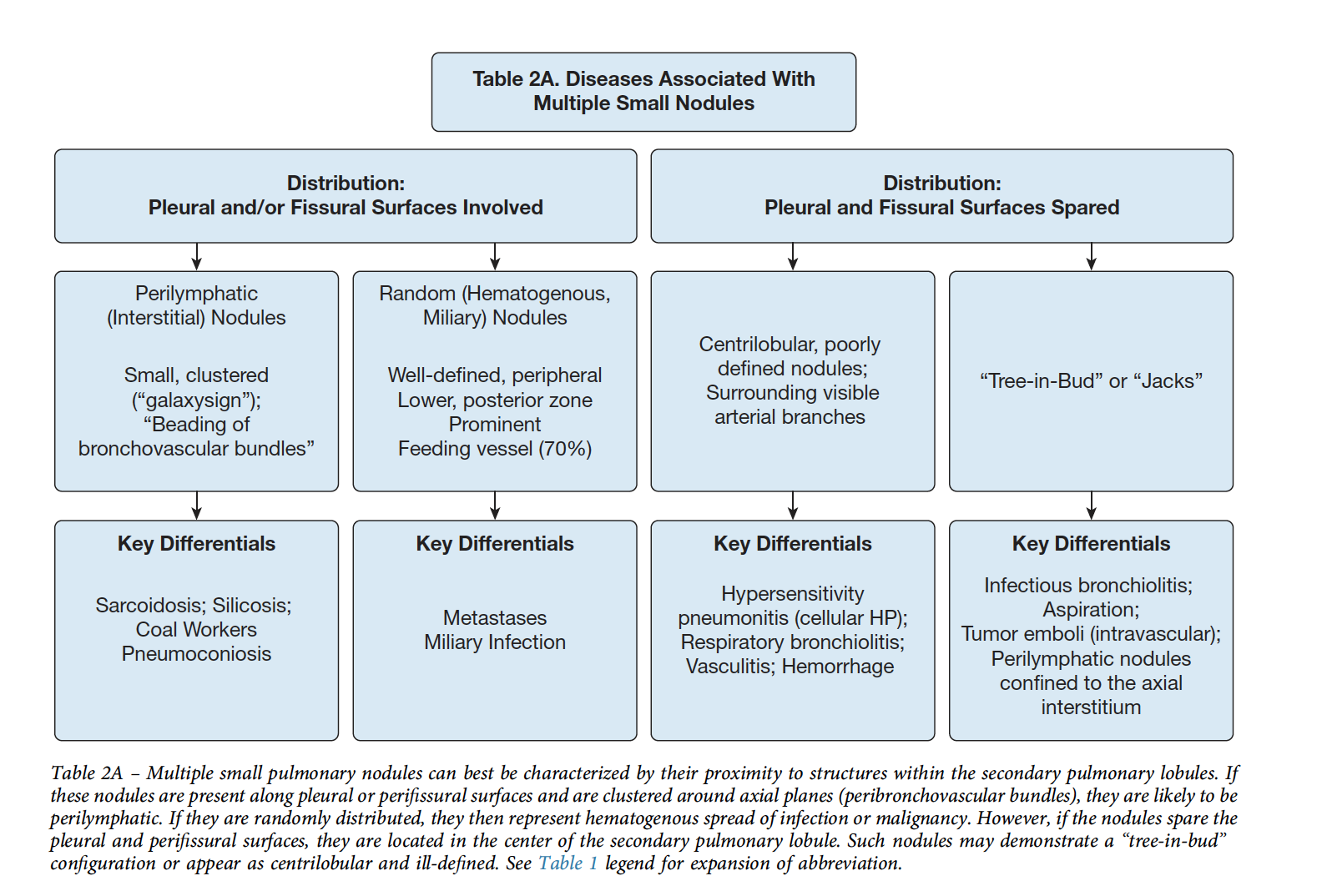
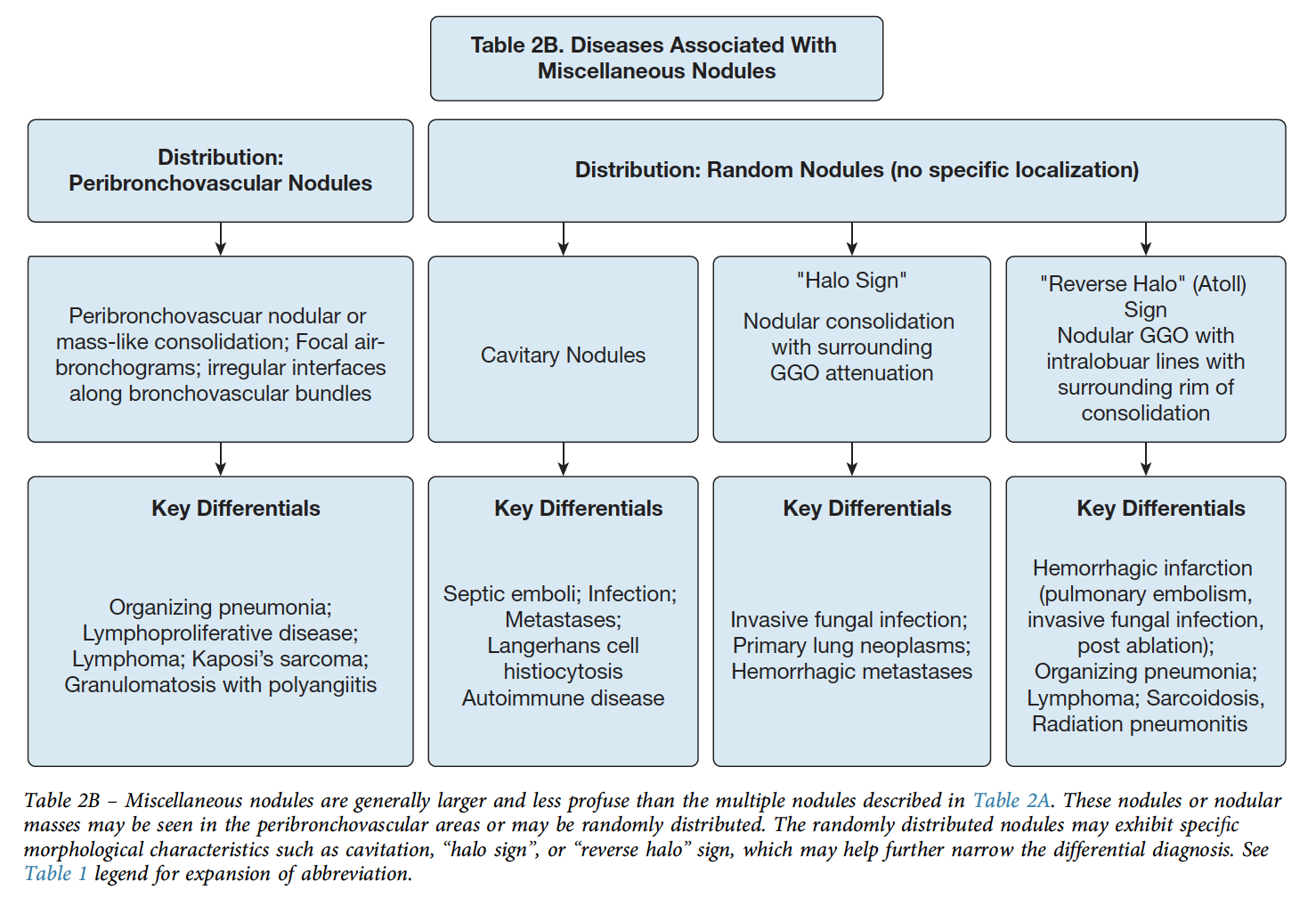
Nodules
Distribution can be:
- Perilymphatic (e.g. subpleural, adjacent to fissures, parahilar, peribronchovascular - and confusingly can be centrilobular): Sarcoidosis, lymphangitic spread, siilicosis, amyloid, LIP.
- Random: miliary infections, hemotogenous mets, sarcoid.
- Centrilobular - most often bronchiolar, though can be related to small vessels less commonly. A subtype of this is Centriolobular Tree-in-bud pattern => dilation and impcation of centrilobular bronchioles due to airway disease. Most commonly, infection, though occasionally asthma w mucus plugging, aspiration, or endobronchial tumor spread.
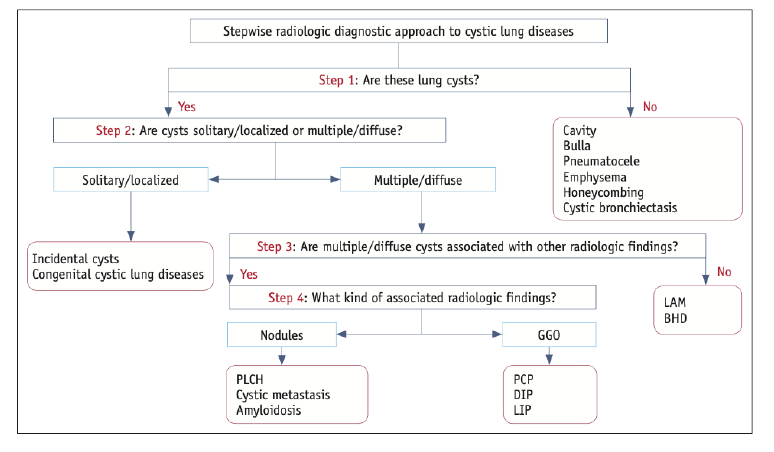
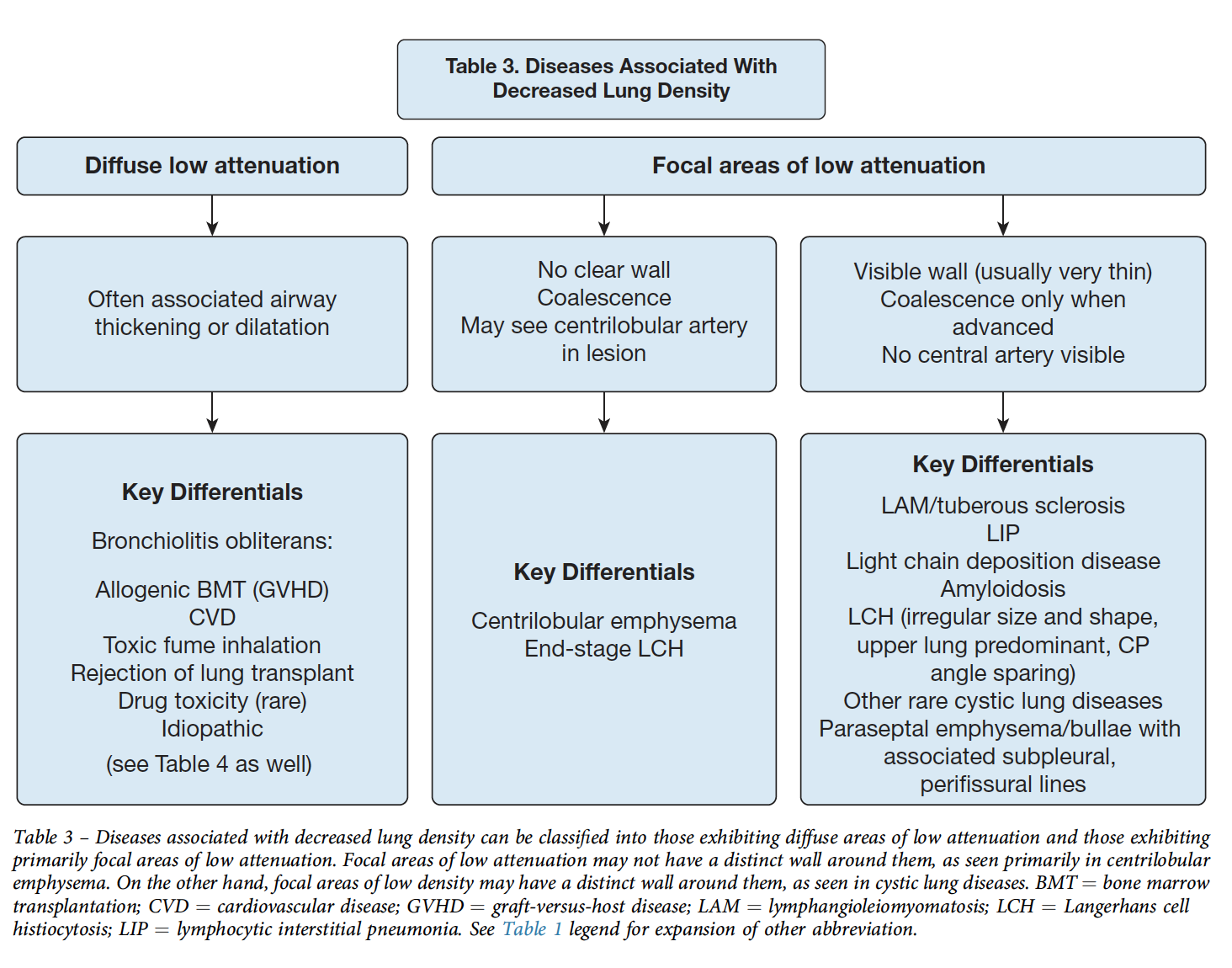
Cystic Lung Disease
[maybe split to separate note]
Note: honeycombing causes cystic spaces, but results from dilation of airways (see above)
Differentiate from cavity = thick walled, irregular shaped (2mm+ thickness)
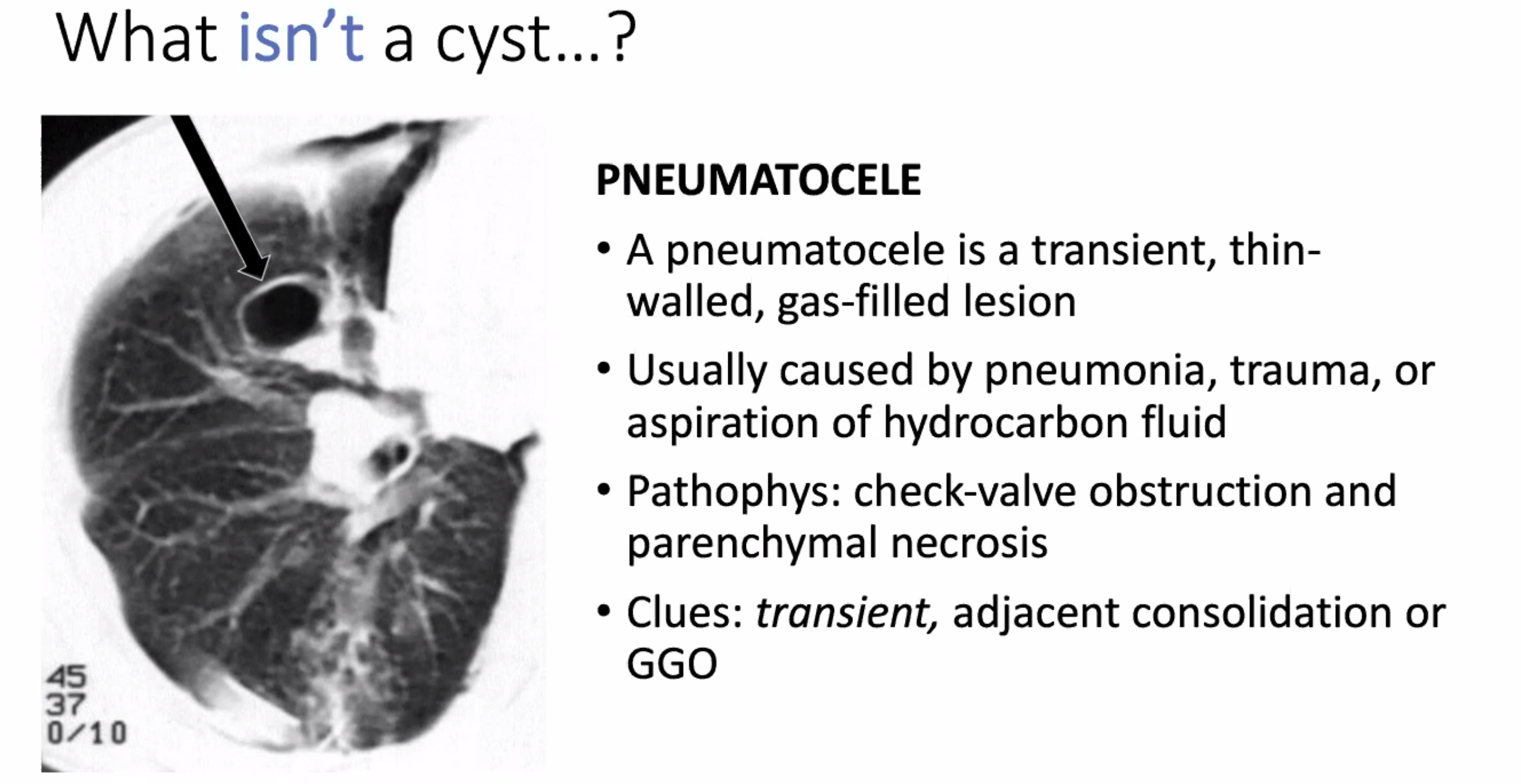
Pneumatocele: transient change from pneumonia or trauma
Congenital
CPAM Intrapulmonary bronchogenic cyst (often mediastinal and fluid filled)
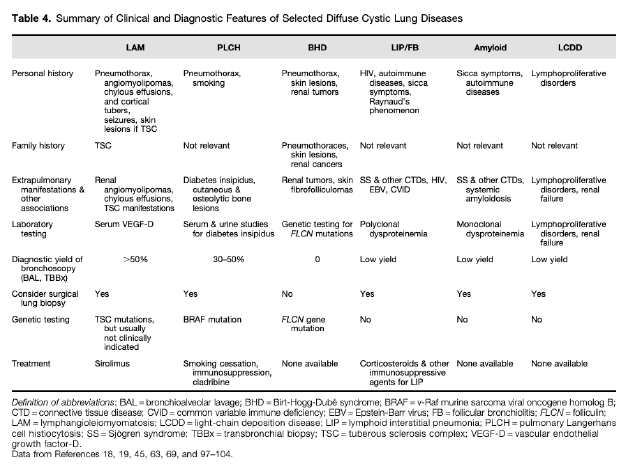
LAM
- not associated with other parenchymal abnormalitieds
- Numerous cysts, small <2cm
- diffuse, random distribution
- Uniform shape
- Avg age of dx 35
- associated with renal AMLs
- Measure serum VegF > 800 (70% sensitive, pathogenesis = smooth muscle cells infiltrate and frustrate angiogenesis)
- treated with Sirolimus. (Often bronchodilators for obstruction, pleurodesis after 1st pneumo due to recurrence risk)
---Differentiate from Birt Hogge Dubbe - more oblong but also have normal parenchyma. Present with pneumothorax. Have fibrosis-folliculomas. Autosomal dominant, high penetrant mutation in Folliculin (tumor suppressor)
PLCH
- Bizarre shaped cysts
- Upper lung predominant, spares costophrenic angles
- Associated with nodules (pathologic accumulations of the langerhan cells: cd1a s-100+, - not known if monoclonal/neoplastic or reactive)
- occurs in smokers, young adults
---differentiate from DIP: can have cysts (30-70%) but mostly within GGOs, lower lobe predominant of cysts
Emphysema
-
paraseptal: near fissures and pleura. More often associated with bulla/blebs
-
centrilobular: no wall, will have some residual tissue in the center of the cystic space 'central dot'. Can become more subtle as the emphysema becomes more extensive (less contrast with normal lung attenuation). Upper lobe predominance. Associated with smoking (airway focused).
-pan lobular (or pan-acinar): uniform destruction of whole secondary lobule (associated with A1AT) - lower lobe, uniform and subtle to differentiate
Bullae and Bleb - accompanied by emphysema in adjacent lung. Same thing just different size. Most often subpleural
Others
LIP - (spectrum with follicular bronchitis). Associated with Sjogrens. Associated with nodules/GGos/Consolidations. Needs surgical biopsy. Associated with MALT lymphoma development.
Amyloidosis / LCDD - most often with lymphoproliferative d/o -> light chain disease
Other malignancy related lung disease
Infections: PJP (technically, pneumatocele), staph, paragonamiasis
Drug Toxicity
Acute Onset
- Acute Lung Injury (DAD): diffuse consolidation, not enlarged pedicle or other signs of interstitial edema -e.g. fissures
- Pulmonary Hemorrhage: bilateral consolidation, may spare subpleural space
- Eosinophilic pneumonia: bilateral consolidation, often acute, transient
Subacute Onset
- (Pathologic) Organizing Pneumonia: bilateral multifocal ground glass attenuating (or consolidated) opacities: perilobular (adjacent to septa), or reverse halo sign. Fibroblastic plugs (which is the key finding on path) in small airways. Can be small nodules
- Diffuse cellular interstitial pneumonitis (lymphocytes/plasma cells) - centrilobular, ill defined, ground glass attenuating.
Drug Related Pneumonitis from Fleischner Society: https://journal.chestnet.org/action/showPdf?pii=S0012-3692%2820%2935313-7
Insidious Onset
- Instititial Fibrosis (UIP, fibrosing NSIP, often unclassifiable). Think of this when there is architectural distortion associated with consolidation.
- Granulomatous Process (can involve lymph nodes) - small nodules in lymphatic distribution and LAD (similar to sarcoid)
Other structures
-Pleural effusions - Drug-induced pleuritis, chylothorax (e.g. desatinib) -Cardiotoxicity - diminished EF to hydrostatic lung edema. -Pulmonary hypertension (group 1)
Radiation Associated Chest Disease
SBRT (=image guided) vs conventional (sometimes used in lymphoma still? - Mantle Radiation)
To tell if it's in field, email the radiation oncologist and ask for the isodose / planning screenshots.
Radiation induced lung injury - 6 weeks after completion of therapy. Show up as focal, poorly marginated, ground-glass or consolidative opacities that evolve to fibrosis (distortion, volume loss, bronchiectasis within the opacitiy aka cicatrization atelectasis and bronchiectasis).
Differentiating recurrence in the field from radiation changes is very hard. Factors making it more likely: sequential enlargement, late enlargement, new spherical opacity, loss of air-bronchogram on subsequent imaging. -FDG-PET is f/u scan to differentiate
Rarer presentations:
- organizing pneumonia outside portal (most common in breast ca)
- recall radiation pneumonitis (e.g. symptoms after initiation of chemo causing findings in previously radiated lung)
- radiation-induced
CT Modes
Maximum intensity projection = MIP: SLAB (vs usual thin slice) and highlighting the highest attenuation value in the slab